Answer :
(a) The mass of water in the 10 mL volumetric flask is, 9.998 g
(b) The density of water in the 10 mL volumetric flask is, 0.9998 g/mL
Explanation :
Part (a) :
Given:
Mass of volumetric flask + Mass of water = 51.436 g
Mass of volumetric flask = 41.438 g
Now we have to calculate the mass of water.
Mass of volumetric flask + Mass of water = 51.436 g
41.438g + Mass of water = 51.436 g
Mass of water = 51.436 g - 41.438 g
Mass of water = 9.998 g
Thus, the mass of water in the 10 mL volumetric flask is, 9.998 g
Part (b) :
Given:
Mass of water = 9.998 g
Volume of water = 10 mL
Now we have to calculate the density of water.
Formula used :
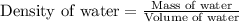
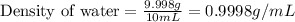
Thus, the density of water in the 10 mL volumetric flask is, 0.9998 g/mL