Answer:
Mass of sodium chloride is needed to prepare 670.g of a 4% sodium chloride solution
26.8 grams
Step-by-step explanation:
Molar mass = It is the amount of substance present in 1 mole of it.
1 mole of substance = Molar mass in grams .\
% mass = Amount of substance present in 100 gram of the solution
4% means 4 gram salt is present in 100 gram of solution
4% of 670 gram is :
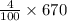
= 26.8 gram
Hence,Mass of sodium chloride is needed to prepare 670.g of a 4% sodium chloride solution is :
26.8 grams
Note :
Molar mass of NaCl = mass of Na + Mass of Cl
= 22.98 + 35.45
= 58.44 g/mol