Answer:
0.2274 mole
Step-by-step explanation:
Calculation of the moles of
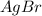 as:-
as:-
Mass = 42.7 g
Molar mass of
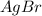 = 187.77 g/mol
= 187.77 g/mol
The formula for the calculation of moles is shown below:
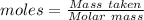
Thus,
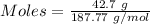
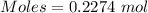
According to the reaction shown below:-
![Na_2S_2O_3 + AgBr\rightarrow NaBr + Na_3[Ag(S_2O_3)_2]](https://img.qammunity.org/2021/formulas/chemistry/high-school/gje81zv214a4ojzffmcwanfd4aontrs1r0.png)
1 mole of
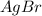 reacts with 1 mole of
reacts with 1 mole of
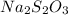
So,
0.2274 mole of
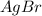 reacts with 0.2274 mole of
reacts with 0.2274 mole of
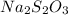
Moles of
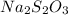 needed = 0.2274 mole
needed = 0.2274 mole