The question is incomplete ,the complete question is:
Titanium(IV) chloride decomposes to form titanium and chlorine, like this:
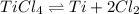
At a certain temperature, a chemist finds that a 5.2 L reaction vessel containing a mixture of titanium(IV) chloride, titanium, and chlorine at equilibrium has the following composition:
Compound amount
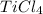 4.18 g
4.18 g
Ti 1.32 g
 1.08g
1.08g
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.
Answer:
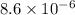 the value of the equilibrium constant for this reaction.
the value of the equilibrium constant for this reaction.
Step-by-step explanation:
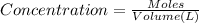
We have : Volume of vessel = 5.2 L
Concentration of Titanium(IV) chloride at equilibrium =:
![[TiCl_4]=(4.18 g)/(190 g/mol* 5.2 L)=0.004231 mol/L](https://img.qammunity.org/2021/formulas/chemistry/college/t5eb5d88b4t1eud2kfo18onh0x5930xe85.png)
Concentration of Titanium at equilibrium =:
![[Ti]=(1.32 g)/(48 g/mol* 5.2 L)=0.005288 mol/L](https://img.qammunity.org/2021/formulas/chemistry/college/g9bosvkeeo4cyz37f3u1yzgp3ng3vqqvmx.png)
Concentration of chloride at equilibrium =:
![[Cl_2]=(1.08 g)/(71 g/mol* 5.2 L)=0.002925 mol/L](https://img.qammunity.org/2021/formulas/chemistry/college/x3zdqie6mqoeixkcz4952wlkx4u6rr4huf.png)
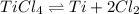
The equilibrium expression will be given as:
![K_c=[Cl_2]^2](https://img.qammunity.org/2021/formulas/chemistry/college/nau8jse1tzkqk0lic3krzef26ymf2ta2ej.png)
The concentration of the solids and liquid is taken as unity.
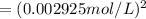
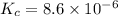
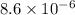 the value of the equilibrium constant for this reaction.
the value of the equilibrium constant for this reaction.