Step-by-step explanation:
The given data is as follows.
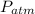 = 98.70 kPa = 98700 Pa,
= 98.70 kPa = 98700 Pa,
T =
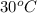 = (30 + 273) K = 303 K
= (30 + 273) K = 303 K
height (h) = 30 mm = 0.03 m (as 1 m = 100 mm)
Density = 13.534 g/mL =
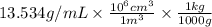
= 13534
The relation between pressure and atmospheric pressure is as follows.
P =
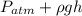
Putting the given values into the above formula as follows.
P =
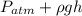
=
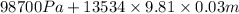
= 102683.05 Pa
= 102.68 kPa
thus, we can conclude that the pressure of the given methane gas is 102.68 kPa.