Answer: The chemical formula of the compound formed is

Step-by-step explanation:
Acids are defined as the chemical species which donate hydrogen ions when dissolved in water.
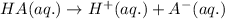
Ionic compound is formed by the complete transfer of electrons from 1 atom to another atom. The cation is formed by the loss of electrons by metals and anions are formed by gain of electrons by non metals.
We are given:
An anion having chemical formula
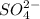 named as sulfate ion
named as sulfate ion
This will combine with 2 hydrogen ions to form a neutral compound. The neutral compound formed is sulfuric acid.
Hence, the chemical formula of the compound formed is
