The question is incomplete, here is the complete question:
A chemistry student needs 10.00 g of isopropenylbenzene for an experiment. She has available 120. g of a 42.7 % w/w solution of isopropenylbenzene in carbon tetrachloride. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button.
Round the answer to 3 significant digits.
Answer: The mass of solution that the student should use is 23.4 grams
Step-by-step explanation:
We are given:
42.7 % (w/w) solution of isopropenylbenzene.
This means that 42.7 grams of isopropenylbenzene is present in 100 grams of solution.
To calculate the mass of solution when 10.00 g of isopropenylbenzene is needed, we apply unitary method:
For 42.7 grams of isopropenylbenzene, the amount of solution needed is 100 grams
So, for 10.00 grams of isopropenylbenzene, the amount of solution needed will be =
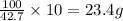
Hence, the mass of solution that the student should use is 23.4 grams