Answer:
Gas state
Step-by-step explanation:
Propane
- Melting point: -190°C
- Boiling point: -42°C
Room temperature: 20°F
We need to calculate the room temperature in °C
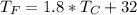


Given that the room temperature is above the propane's boiling point it is in gas state