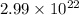Answer:
grams of zinc required = 3.24 g
Particles of zinc required =
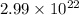
Step-by-step explanation:
Given that:-
Mass of hydrogen gas produced = 0.10 g
Calculation of the moles of
 as:-
as:-
Mass = 0.10 g
Molar mass of
 = 2.016 g/mol
= 2.016 g/mol
The formula for the calculation of moles is shown below:
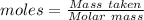
Thus,
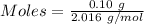
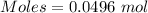
According to the reaction shown below:-
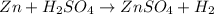
1 mole of hydrogen gas is produced when 1 mole of zinc reacts
So,
0.0496 mole of hydrogen gas is produced when 0.0496 mole of zinc reacts
Moles of Zinc required = 0.0496 moles
Molar mass of zinc = 65.38 g/mol
mass= Moles*Molar mass =
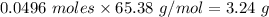
Also, 1 mole of Zinc contains
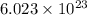 particles
particles
0.0496 mole of Zinc contains
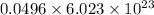 particles
particles
Particles of zinc required =