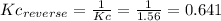Step-by-step explanation:
1)
 + 7 H_2(g)](https://img.qammunity.org/2021/formulas/chemistry/college/maek8n2ul1kpucg4baukg4xxz3sqga0bvz.png)
![Kc=([Na[Al(OH)_4]]^2*[H_2]^7)/([NaOH]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/nxe9rztchgqs73kkukjwomioypbtv32dna.png)
The Kc for the reverse reaction is the inverse of the Kc of the reaction:
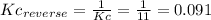
2)
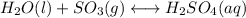
![Kc=([H_2SO_4])/([SO_3]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/wawnepkjla8nn3al7od7f4bzeb1p5683jp.png)
The Kc for the reverse reaction is the inverse of the Kc of the reaction:
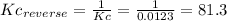
3)
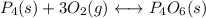
![Kc=(1)/([O_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/3wvpltfb0h4hm9xuukpqf8mp148u51nsoo.png)
The Kc for the reverse reaction is the inverse of the Kc of the reaction: