Answer : The molarity and molality of the student's solution is, 0.12 mol/L and 0.12 mol/kg respectively.
Explanation : Given,
Density of solvent = 1.01 g/mL
Molar mass of sucrose = 342.3 g/mole
Mass of sucrose = 12 g
Volume of solvent = 300 mL
First we have to calculate the mass of solvent.
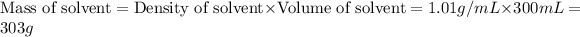
Now we have to calculate the molarity of solution.
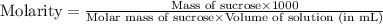
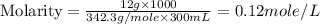
Now we have to calculate the molality.
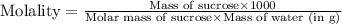
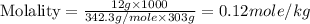
Therefore, the molarity and molality of the student's solution is, 0.12 mol/L and 0.12 mol/kg respectively.