Answer:
The molecular formula for diprotic acid is :
a)- C27H19012
Step-by-step explanation:
It is given in the question that :
The titration requires 25 mL of 0.850M NaOH to neutralize 5.692g of the acid.
1.Calculate Moles of NaOH,
M = 0.850 M
V = 25 mL
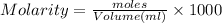
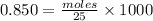
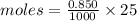
Moles of NaOH = 0.02125
2. Calculate the moles of Acid
But, moles of diprotic acid = moles of NaOH
Since it is a diprotic acid it require 2 mole of NaOH (Diprotic gives 2H+)
So,
Moles of Acid required for neutralisation = 1/2 moles of NaOH
moles of Acid =
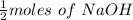
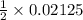
Moles of Acid = 0.010625
3.Calculate the molar mass of Acid
Mass = 5.692 g
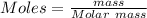
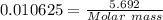
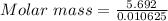
Molar mass = 535.71 g
4.Check from the options which has Molar mass = 535 gram or around
a)C27H19012
= 27(mass of C) + 19(mass of H) + 12(mass of O)
= 27(12) + 19(1) + 12(16)
= 324 + 19 + 192
= 535 gram
Hence , A is correct option.