Answer:
pH = 2.77
Step-by-step explanation:
Data Given:
degree of dissociation Ka = 0.057
Concentration of Methanoic acid = 0.005 M
pH = ?
Solution:
First we calculate Hydrogen ion concentration
Formula will be used
[H⁺] =
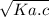 . . . . . . (1)
. . . . . . (1)
where
Ka = degree of dissociation
c = concentration
Put values in formula 1
[H⁺] =
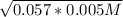 . . . . . . (1)
. . . . . . (1)
[H⁺] = 0.0017 M
Now Calculate the pH
As we know
pH = -log [H⁺] . . . . . .(2)
Put values in equation
pH = - log [ 0.0017 M]
pH = - [-2.77]
pH = 2.77
So,
pH of methanoic acid = 2.77