Answer:
A general form of a reaction type tells you what the reaction basically looks like. It typically uses letters like "A" and "B" to represent an element.
Learning the general forms help you recognize reactions when there are actual elements.
Step-by-step explanation:
The six types of reactions are synthesis, decomposition, single displacement, double displacement, combustion of hydrocarbons, and neutralization. Here are their general forms:
Synthesis
A + B => AB
It shows you that when you have substance A and substance B, they will become one compound after the reaction. It's the opposite for decomposition.
Decomposition
AB => A + B
It shows you that when you have compound AB, it will become two separates elements after the reaction. It's the reverse of synthesis.
Single displacement
AB + C => AC + B
One of the elements in a compound in the reactant (left) side was replaced after the reaction by a new element.
Double displacement
AB + CD => AC + BD
Each of the compounds had one element replaced by a new element from the other compound.
Combustion of hydrocarbons
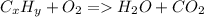
You can see that there will always be a hydrocarbon (hydrogen (H) and carbon (C) compound) in the reactants. When it reacts with oxygen, it will combust, producing water and carbon dioxide.
Neutralization
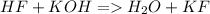
In neutralization, there must be one compound with H (an acid) and a compound with OH (a base). When they react, they produce water and "KF", which is called a "salt".