Answer:
They meet at the distance of 32.5 cm
Step-by-step explanation:
The rate of diffusion is inversely proportional to the molar mass of gases.
If diffusion is represented as d and mass of gases as M. Then , rate is given as:
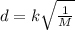
For , two gases
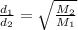
First , Calculate the ,molar mass of each gas :
CH4 = 1 (mass of C) + 4(mass of H)
= 1(12)+4(1)
= 12 + 4 = 16 gram
M1 = 16 gram
C4H8 = 4(mass of C) + 8(Mass of H)
= 4(12) + 8(1)
= 48 + 8
M2 = 56 grams
d1 is diffusion of CH4
d2 = diffusion of C4H8
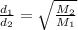
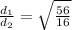 }
}
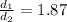
This means CH4 diffuse 1.87 times faster than C4H8
Now, the mathematical part
If velocity of C4H8 = 1 cm/sec , then velocity of CH4 = 1.87 cm/sec
v1 = 1.87 cm/sec
v2 = 1 cm/sec
Let both meet at time "t". If distance travelled by CH4(s1) = x , then remaining(s2) distance (50-x) is travelled by C4H8
(see the attached image)
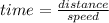
since both meet at same time so t = constant.
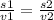
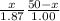
on solving for x we get,
x = 17.42 cm
from the other side the distance is (50 - x)
= 50 - 17.42 = 32.5 cm