Answer:
The balanced reaction is :
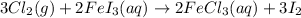
Iodine produce = 20.92 grams
Ferric Chloride produced = 8.92 grams
Step-by-step explanation:
Molar mass : The amount of sustance present in 1 mole of it.
1 mole = Molar mass of the substance.
1 mole of Cl2 = 71 gram
3 mole of Cl2 = 71 x 3 = 213 gram
1 mole of I2 = 253.8 gram
3 mole of I2 = 3 x 253.8 = 761.4 gram
1 mole of FeCl3 = 162.2 gram
2 mole of FeCl3 = 2 x 162.2 = 324.4 gram
1 mole of FeI3 = 436.56 gram
2 mole of FeI3 = 2 x 436.56 gram =873.12
(use periodic table for calculation)
According to the given question 12 gram of Cl2 reacts with 24 gram of FeI3
The balanced equation is :
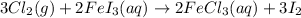
Here
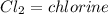
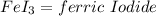
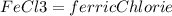
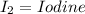
This shows:
3 mole Cl2 = 2 mole FeI3 = 2 mole FeCl3 = 3 mole I2
first ,calculate the limiting reagent (the substance present in less quantity)
3 mole Cl2 = 2 mole FeI3
213 gram of Cl2 = 873.12 gram of FeI3
1 gram =
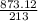
12 gram of Cl2 will give =
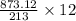
= 49.18 gram of FeI3 is needed
FeI3 present = 25 gram
So, FeI3 is limiting reagent as it is less than needed
Now , Find the amount of Products with 24 gram of FeI3 because it is the limiting reagent
1. To calculate FeCL3 produced
2 mole FeI3 = 2 mole FeCl3
873.12 gram of FeI3 = 324.4 gram of FeCl3
1 gram =
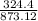
24 g FeI3 =
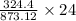 of FeCl3
of FeCl3
= 8.92 gram of FeCl3
2. To calculate I2
2 mole of FeI3 = 3 mole I2
873.12 gram of FeI3 = 761.4 gram of I2
24 gram of FeI3
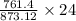
= 20.92 gram of I2