
Here's the required chemical equation for given reaction ~
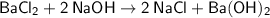
1 mole of Barium chloride reacts with 2 moles of Sodium hydroxide to form 2 moles of Sodium Chloride and 1 mole of Barium hydroxide.
Note : There can be a reaction with some different values or moles, but the proportion should be same