Answer:
To find a base by its formula, does it end in COO-
A base never ens with COOH
Step-by-step explanation:
According to Bronsted Acid - Base theory : When an acid and base reacts with each other , the acid form its conjugate base and base will form its conjugate acid because of exchange of proton .
If HA = acid and B = base react each other then they generate their conjugate Base (A-) and acid (HB+) respectively .
The equation is represented as :
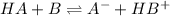
Here A- is conjugate base of HA acid
HB+ is conjugate acid of base B
COOH group is the carboxylic acid group = acid
Suppose A carboxylic acid RCOOH is added in water(H2O)
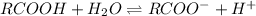
Here
RCOOH = Acid and its conjugate -base is RCOO-
Hence A Base end with formula COO-
COOH is always acid