Reaction 2 will be 1.621 times faster than reaction 1
Step-by-step explanation:
As the reaction 2 is taking 0.470 mol /L and reaction 1 is taking 0.290 mol /L, then the ratio of the rate of reaction will be
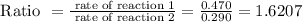
Asked reaction 2 how much faster than reaction 1, so rearrange the above equation, we get,
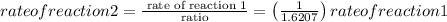
Thus, 1.621 times reaction 2 is faster than reaction 1 as per the given question. As the reaction 2 is using more amount of reactants compared to reaction 1, the reaction 2 will be 1.6207 times faster than reaction 1.