Answer:

Step-by-step explanation:
Given a reaction mechanism, we will typically have catalysts and intermediates which will not be observed in the final balanced overall reaction. In order to obtain a net reaction, we need to add all the separate steps of the given reaction.
First of all, the reactants are combined followed by a combination of products. The repeating species on both sides of the final equation are then canceled out. Let's sum everything we have in our steps:
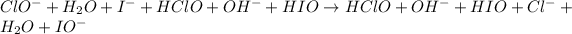
Repeating species that can be canceled out are:
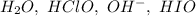
This leaves a net reaction of:
