Answer:
8.467 gm
Step-by-step explanation:
The law governing this problem is "The Law of Constant Composition "
As per this law, all compounds irrespective of their origin and source have the same composition and properties in their purest form
It is a simple proportion and ratio related problem.
1.50 grams of carbon require 2.0 grams of oxygen
1.0 grams of carbon will require oxygen
=
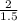
6.35 grams of carbon will require oxygen
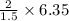 = 8.4666\\= 8.467
= 8.4666\\= 8.467