Answer:
The answer to your question is percent yield = 85.6
Step-by-step explanation:
Data
mass of Na = 58.3 g
mass of Br = 54.1 g
mass of NaBr = 59.6 g
Reaction
2Na(s) + Br₂ ⇒ 2NaBr (s)
Process
1.- Calculate the atomic mass of the reactants and products
sodium = 2 x 23 = 46 g
bromine = 2 x 80 = 160 g
NaBr = 2(23 + 80) = 206 g
2.- Calculate the limiting reactant using proportions
Theoretical Na / Br = 46 / 160 = 0.2875
Experimental Na / Br = 58.3 / 54.1 = 1.077
From these data we conclude that the limiting reactant is Br, because the proportion was higher in the experiment than means that there was less Bromine or more Sodium.
3.- Calculate the theoretical production of NaBr
160 g of Br --------------- 206 g of NaBr
54.1 g of Br --------------- x
x = (54.1 x 206) / 160
x = 11144.6 / 160
x = 69.65 g of NaBr
4.- Calculate the percent yield
% yield =
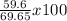
% yield = 85.6