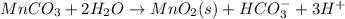Answer:
The half-reaction for the oxidation of the manganese in
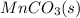 to
to
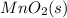 .:
.:
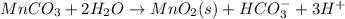
Step-by-step explanation:
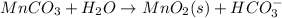
Let us say the medium in which reaction is taking place is an acidic medium. And balancing in an acidic mediums done as;
Step 1: Balance all the atom beside oxygen and hydrogen atom;
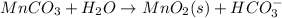
Manganese and carbon are balanced.
Step 2: Balance oxygen atom adding water on the required side:
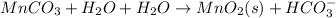
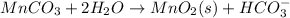
Step 3: Now balance hydrogen atom by adding hydrogen ion on the required side: