Step-by-step explanation:
Balanced chemical reaction equation will be as follows.
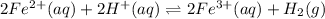
In human body, the neutral iron changes into
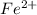 (aq) cation. There will be an oxidation-half reaction and a reduction-half reaction. Equations for this reaction are as follows.
(aq) cation. There will be an oxidation-half reaction and a reduction-half reaction. Equations for this reaction are as follows.
Oxidation: 2Fe^{2+}(aq) \rightleftharpoons 2Fe^{3+}(aq) + 2e^{-}[/tex] .... (1)
Reduction:
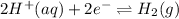 ...... (2)
...... (2)
On adding both equation (1) and (2), the overall reaction equation will be as follows.
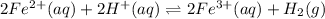
Therefore, neutral iron is a part of Heme - b group of Hemoglobin and in an aqueous solution it dissolutes as a part of Heme group. Hence, then it becomes an
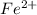 cation.
cation.