Answer:
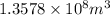 volume of carbon dioxide gas would be collected during a one-year period.
volume of carbon dioxide gas would be collected during a one-year period.
Step-by-step explanation:
Mass of carbon dioxide gas collected in a day = 31,000 Tonn
1 Tonn = 1000 kilogram
31,000 Tonn =
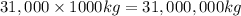
Specific volume of the carbon dioxide gas =
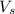
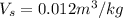
Volume of carbon dioxide gas collected in a day = V
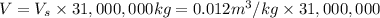
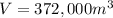
1 year = 365 days
Volume of carbon dioxide gas collected in a year = V'
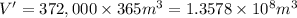
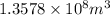 volume of carbon dioxide gas would be collected during a one-year period.
volume of carbon dioxide gas would be collected during a one-year period.