Answer:
The aqueous solution of
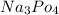 therefore would be "strongly basic."
therefore would be "strongly basic."
Step-by-step explanation:
Strongly basic because the
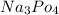 is completely an ionic compound. These compound can be 100% splitted into metal ions and hydroxide ion when used in the aqueous solution. Each moles dissolves which can give rise to mole of hydrogen ions when used in the solution. Whereas strong bases are not soluble in water as well. in the presence of water equilibrium can also be achieved. A strong base has the capability which can just remove the proton from the molecule.
is completely an ionic compound. These compound can be 100% splitted into metal ions and hydroxide ion when used in the aqueous solution. Each moles dissolves which can give rise to mole of hydrogen ions when used in the solution. Whereas strong bases are not soluble in water as well. in the presence of water equilibrium can also be achieved. A strong base has the capability which can just remove the proton from the molecule.