Answer:
The volume of the gas at constant temperature is:
186.16 mL
Step-by-step explanation:
Byole's Law : The pressure exerted by the fixed amount of gas is inversely proportional to Volume at the constant temperature.

Here k = proportionality constant

If P1 and V1 are initial pressure and Volume of gas and P2 , V2 are final volume of the gas , Then :
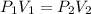
P1 = 542.48 mm Hg
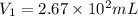
P2 = 778.05 mm Hg
Insert the values of P1 , V1 and P2 in formula and calculate V2
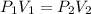

dividing the equation by 778.05



