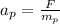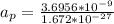To solve this exercise, we will first proceed to calculate the electric force given by the charge between the proton and the electron (it). From the Force we will use Newton's second law that will allow us to find the acceleration of objects. The Coulomb force between two charges is given as
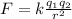
Here,
k = Coulomb's constant
q = Charge of proton and electron
r = Distance
Replacing we have that,
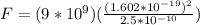
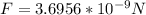
The force between the electron and proton is calculated. From Newton's third law the force exerted by the electron on proton is same as the force exerted by the proton on electron.
The acceleration of the electron is given as
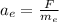
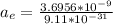
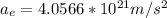
The acceleration of the proton is given as,