Answer:
2.73 M
Step-by-step explanation:
The balanced reaction that takes place is:
MnO₄⁻ + 8H⁺ + 5Fe²⁺ → Mn²⁺ + 5Fe⁺³ + 4H₂O
Moles of MnO₄⁻ that reacted = 0.265 M * 38.65 mL = 10.24 mmol MnO₄⁻
Moles of Fe²⁺ that reacted = 10.24 mmol MnO₄⁻ *
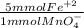 = 51.21 mmol Fe²⁺
= 51.21 mmol Fe²⁺
Molarity of Iron (II) = 51.21 mmol Fe²⁺ / 18.78 mL =2.73 M