Answer: Option (e) is the correct answer.
Step-by-step explanation:
Chemical formula of a nitrate ion is
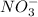 and it acts as a strong oxidizing agent because it itself gets reduced easily.
and it acts as a strong oxidizing agent because it itself gets reduced easily.
As we know that an oxidizing agent tends to oxidize other substances by itself gaining electrons. As a result, there will occur a decrease in its oxidation state.
The oxidation number of nitrogen in
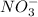 is +5. So, it gains electrons and helps in oxidizing other substances.
is +5. So, it gains electrons and helps in oxidizing other substances.
Thus, we can conclude that the nitrate anion is a strong oxidizing agent.