The question is incomplete , the complete question is ;
In a study of the following reaction :
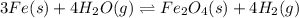
at 1200 K it was observed that when the equilibrium partial pressure of water vapor is 15.0 torr, the total pressure at equilibrium is 36.3 torr. Calculate the
 foe this reaction at 1200 K.
foe this reaction at 1200 K.
Answer:
The
 for this reaction at 1200 K is 4.066[/tex].
for this reaction at 1200 K is 4.066[/tex].
Step-by-step explanation:
Partial pressure of the water vapor at equilibrium =
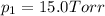
Partial pressure of the hydrogen gas at equilibrium =
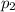
Total pressure at equilibrium =
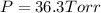
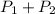
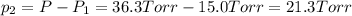
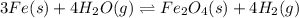
The expression if
 is given as;
is given as;
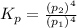
(the partial pressure of the gas will be taken along with which partial pressure of the solids are taken as unity)
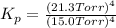
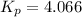
The
 for this reaction at 1200 K is 4.066[/tex].
for this reaction at 1200 K is 4.066[/tex].