Answer:
In this reaction heat is released
Step-by-step explanation:
Exothemic Reactions : Those reactions in which heat is released when the products are formed .
For such reaction The enthalpy change is negative.
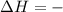 for exothermic reaction
for exothermic reaction
Endothermic Reactions : Those reaction which proceed by absorbing heat . Here heat is absorbed. The enthalpy change for such reactions is positive.
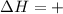
When the direction of reaction reverses , the value of H also changes.
In the given reaction,
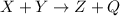
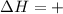 = heat is absorbed
= heat is absorbed
So,
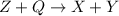
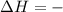 = heat is released
= heat is released
Exothermic reactions are more spontaneous as compared to endothermic ractions. This means that they proceed own their own . Only little amount of energy is supplied to exothermic reaction to produce the prodct.