This is an incomplete question, here is a complete question.
Raising the temperature of 10.0 g of water from 10.0 °C to 20.0 °C requires 100.0 cal of energy, while raising the temperature of 10.0 g of aluminum from 10.0 °C to 20.0 °C requires 22 cal. More calories are required to heat the water because:
(1) water is a liquid and aluminum is a solid at 10.0 °C.
(2) 10.0 °C is closer to the melting point of water than to the melting point of aluminum.
(3) water has a greater potential energy than aluminum.
(4) ten grams of water occupies a larger volume than 10.0 g of aluminum.
(5) water has a larger specific heat than aluminum.
Answer : The correct option is, (5) water has a larger specific heat than aluminum.
Explanation :
Specific heat capacity : It is defined as the amount of heat absorbed by one gram of a substance to raise its temperature by one degree Celsius.
Formula used for specific heat capacity :
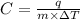
where,
C = specific heat capacity
m = mass of a substance
q = heat required
 = change in temperature of substance
= change in temperature of substance
The heat required is depend on the mass, specific heat and temperature of substance.
As per question, the mass and raising temperature of water and aluminum is same but it requires different energy to heat.
More calories are required to heat the water because water has a larger specific heat than aluminum.
Hence, the correct option is, (5)