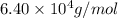Answer : The molar mass of hemoglobin is,
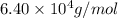
Explanation : Given,
Molar mass of iron = 55.85 g/mol
0.349 % Fe by mass that means 0.349 grams of Fe present in 100 grams of hemoglobin.
or,
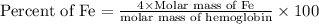
Now put all the given values in this formula, we get:
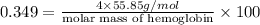
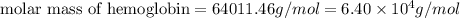
Thus, the molar mass of hemoglobin is,