Answer:
At pH 8.59 the precipitation of acid will form.
Step-by-step explanation:
The pH below which the drug will begin to precipitate can be calculated using the relation
pH =
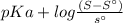
where S = total saturation solubility of the drug
S° = solubility of the undissociated species
substituting the respective values in equation, we get:
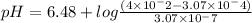
pH = 8.59