Answer:
2.13 moles of AlCl3 are produced from 3.2 moles of Cl2
Step-by-step explanation:
Moles : The amount of substance that contain as many particles as present in 12 grams of C-12.
The balanced equation for the reaction is :
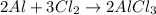
This reaction shows the following :
2 mole Al = 3 mole Cl2 = 2 mole AlCl3
In question we are asked to calculate the moles of AlCl3 produced from Cl2
So, we will use only Cl2 andAlCl3 moles
3 mole Cl2 = 2 mole AlCl3
1 moles of Cl2 will give =
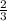 of AlCl3
of AlCl3
3.2 moles of Cl2 will give =
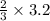 of AlCl3
of AlCl3
= 2.133 moles