Answer:
The correct answer are option B and D.
Step-by-step explanation:
ΔG° = ΔH° - TΔS°
Where:
ΔG° = Gibbs's free energy of the reaction at temperature T.
ΔH° = Enthalpy of the reaction at temperature T.
ΔS° = Change in entropy of the reaction at temperature T.
- If the vales of Gibb's free energy is less than zero or negative than the reaction is said to be spontaneous that is feasible under given conditions.
- If the vales of Gibb's free energy is greater than zero or positive than the reaction is said to be non spontaneous that is non feasible under given conditions.
From the given reaction, the reaction with value of
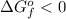 represents the a feasible way to synthesize the product. So, the correct answer are option B and D.
represents the a feasible way to synthesize the product. So, the correct answer are option B and D.