Answer: The sample contains NaCl(s) and LiCl(s).
Step-by-step explanation:
1 mole of NaCl contains 1 mole of sodium atom and 1 mole of chlorine atom
1 mole of LiCl contains 1 mole of lithium atom and 1 mole of chlorine atom
To calculate the percentage composition of chlorine in sample, we use the equation:
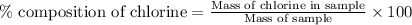
Mass of NaCl = 58.5 g/mol
Mass of Chlorine atom = 35.5 g/mol
Putting values in above equation, we get:
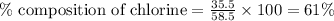
Mass of LiCl = 42.5 g/mol
Mass of Chlorine atom = 35.5 g/mol
Putting values in above equation, we get:
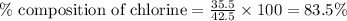
Taking the average of mass percent of chlorine in both the compounds:
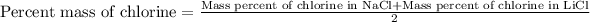
Putting values in above equation, we get:

Hence, the sample contains NaCl(s) and LiCl(s).