Answer : The value of
 of the reaction is 10.5 and the reaction is product favored.
of the reaction is 10.5 and the reaction is product favored.
Explanation : Given,
Moles of
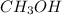 at equilibrium = 0.0406 mole
at equilibrium = 0.0406 mole
Moles of
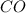 at equilibrium = 0.170 mole
at equilibrium = 0.170 mole
Moles of
 at equilibrium = 0.302 mole
at equilibrium = 0.302 mole
Volume of solution = 2.00 L
First we have to calculate the concentration of
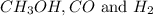 at equilibrium.
at equilibrium.
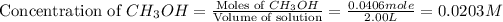
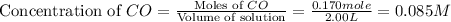
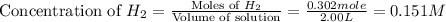
Now we have to calculate the value of equilibrium constant.
The balanced equilibrium reaction is,
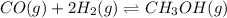
The expression of equilibrium constant
 for the reaction will be:
for the reaction will be:
![K_c=([CH_3OH])/([CO][H_2]^2)](https://img.qammunity.org/2021/formulas/chemistry/high-school/221676sk33k3xtvein3nbay5z2mn8nt4yi.png)
Now put all the values in this expression, we get :
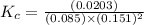

Therefore, the value of
 of the reaction is, 10.5
of the reaction is, 10.5
There are 3 conditions:
When
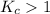 ; the reaction is product favored.
; the reaction is product favored.
When
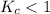 ; the reaction is reactant favored.
; the reaction is reactant favored.
When
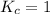 ; the reaction is in equilibrium.
; the reaction is in equilibrium.
As the value of
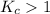 . So, the reaction is product favored.
. So, the reaction is product favored.