Answer:
ΔT = -92°C
Step-by-step explanation:
given,
mass of silver = 35 g
heat = 180 cal
temperature change = ?
The specific heat capacity of silver is 0.056 cal/g C
using heat formula for calculation of temperature.
Q = m Cp ΔT
180 = 35 x 0.056 x ΔT
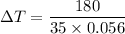
ΔT = -92°C
negative sign is used because silver is cooled and temperature change is from high temperature to low.