Answer:
Molar Mass=193.9738 g/mol
Molar Mass≅194g/mol
Step-by-step explanation:
Consider the formula:
m=ΔT/K_{f}
where:
ΔT is freezing point depression
K_{f} is freezing point depression constant
m is the morality=(moles of solute/kg of solvent)
Now:
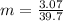
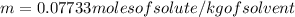
Now:
0.07733 (moles of solute/kg of solvent) *0.010 kg of solvent
 moles of solute(Caffeine)
moles of solute(Caffeine)
Molar mass = Mass of solute/moles of solute
Molar Mass=
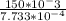
Molar Mass=193.9738 g/mol
Molar Mass≅194g/mol