Answer:
3 moles of hydrogen,
 , are needed to react completely with 1 moles of nitrogen,
, are needed to react completely with 1 moles of nitrogen,

Step-by-step explanation:
Let us consider the Reaction of Nitrogen and hydrogen
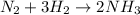
Two nitrogen atom reacts with 3 hydrogen atom to form ammonia. So it is clear from the chemical equation that 3 moles of hydrogen atom is essential to react completely with nitrogen.
Also when we see the atomic structure of hydrogen and nitrogen ,Hydrogen has only one electron its shell and nitrogen has 5 electron in its valency shell. so for an Nitrogen to have a completely filled shell, it needs 3 more electrons .Thus is need 3 hydrogen atom