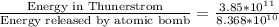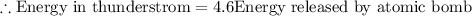To solve this problem we will define the values given for the latent heat required by condensation the net mass of water and apply the concept of heat released. Later we will compare the two values.
Latent heat required for condensation is
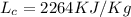
Air contain water:
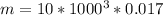
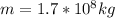
Now the heat released is
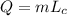
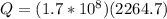
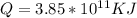
Using the value of energy released by atomic bomb, which is
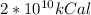 and converting in Jules,
and converting in Jules,
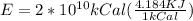
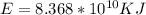
Compariong we have that,