Answer:
20.98 gram of CO
Step-by-step explanation:
Limiting Reagent : The reactant which is present in less amount and get consumed after the reaction completes.
Molar mass : Mass of the substance present in 1 mole of the compound.
Molar mass of Fe = 55.84 gram/mole
1 mole Fe = 55.84 g
2 mole of Fe = 2 x 55.84 g = 111.68 g..........(1)
mass of C = 12
Mass of O = 16
Molar mass of CO = mass of C + mass of O
Molar mass of CO = 28 gram
1 mole of CO = 28 g
3 mole of CO = 3 x 28 = 84 g..........(2)
We need to know which is limiting reagent to calculate the amount of product or vice-versa.
In this question , it is already given that Fe2O3 is excess reagent .Hence CO must be the limiting reagent.
The balanced equation is :
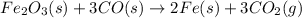
this equation indicates ,
3 moles of CO produce = 2 mole of Fe
or in other words,
2 moles of Fe are produced from = 3 moles of CO
111.68 gram of Fe is produced from = 84 gram of CO
(from equation (1) and (2))
So ,
1 gram of Fe is produced from =
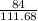 of CO
of CO
27.9 g of Fe is produced from=
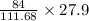 gram of CO
gram of CO
= 20.98 gram of CO
= 21 gram of CO (In round figures)