Answer:
16.19 days.
Explanation:
The radioactive iodine is used to determine the health of the thyroid gland and it decays according to the equation
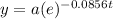 where t is in days and a is the initial amount and y is the final amount after decay for t days.
where t is in days and a is the initial amount and y is the final amount after decay for t days.
We have to find a one-fourth life of this substance.
So,
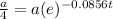
⇒
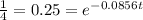
Now, taking ln both sides we get,
ln (0.25) = - 0.0856t ln e {Since,
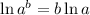 }
}
So, t = 16.19 days. (Answer)