Answer:
422455.41
Step-by-step explanation:
Corrected from source,
Given that:-
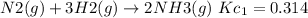
The equilibrium constant for the reverse reaction will be the reciprocal of the initial reaction.
The value of equilibrium constant the reaction
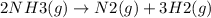
is:
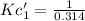
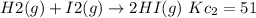
If the equation is multiplied by a factor of '3', the equilibrium constant of the reverse reaction will be the cube of the equilibrium constant of initial reaction.
The value of equilibrium constant the reaction
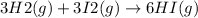
is:
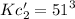
Adding both the reactions we get the final reaction. So, the equilibrium constants must be multiplied.
The value of equilibrium constant the reaction
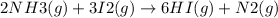
is:
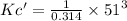 = 422455.41
= 422455.41