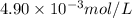The question is incomplete, here is the complete question.
A chemist prepares a solution of copper(II) fluoride by measuring out 0.0498 g of copper(II) fluoride into a 100.0mL volumetric flask and filling the flask to the mark with water.
Calculate the concentration in mol/L of the chemist's copper(II) fluoride solution. Round your answer to 3 significant digits.
Answer: The concentration of copper fluoride in the solution is
Step-by-step explanation:
To calculate the molarity of solute, we use the equation:
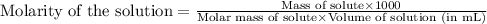
We are given:
Given mass of copper (II) fluoride = 0.0498 g
Molar mass of copper (II) fluoride = 101.54 g/mol
Volume of solution = 100.0 mL
Putting values in above equation, we get:
![\text{Molarity of copper (II) fluoride)=(0.0498* 1000)/(101.54* 100.0)\\\\\text{Molarity of copper (II) fluoride}=4.90* 10^(-3)mol/L]()
Hence, the concentration of copper fluoride in the solution is