Answer:
44.46 years
Step-by-step explanation:
Let water fusion heat at atmospheric environment be f = 333550 j/kg
218km = 218000m
25 km = 25000m
a) The iceberg volume is its width times length times thickness:

The mass of the iceberg is its density times volume
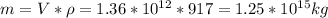
b) The heat energy required to transform the iceberg from solid to liquid form is
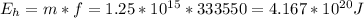
c) Suppose only the upper surface is subjected to sunlight, then we can calculate the sun light area, which is length times width
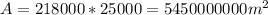
Then the sunlight power, or energy per unit of time that is being transferred to that surface of the iceberg is
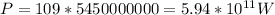 or J/s
or J/s
The time it needs (in seconds) to melt:
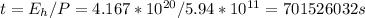
or 701526032 / (60*60) = 194868 hours
As each day only has 12 hours of sunlight, the number of days it'd need is
194868 / 12 = 16239 days or 16239/365.25 = 44.46 years