Answer:
pkb =2.92
Step-by-step explanation:
pH is defined as the negative logarithm of the concentration of hydrogen ions.
Thus,
pH = - log [H⁺]
Given that:- pH = 12.04
Also, pH + pOH = 14
So, pOH = 14 - 12.04 = 1.96
The expression of the pOH of the calculation of weak base is:-
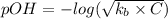
Where, C is the concentration = 0.1 M
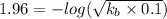
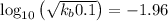
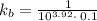
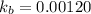
pkb = -log kb =
 = 2.92
= 2.92