Answer:
For the Ag ground-state ion, the type of orbital from which an electron will need to be removed to form the ion of greater positive charge is :
s-orbital
Step-by-step explanation:
Naming of orbitals :
s = s-orbital
p = p-orbital
d = d-orbital
The electronic configuration of Ag is :
![[Kr]4d^(10)5s^(1)](https://img.qammunity.org/2021/formulas/chemistry/college/vs7thl5gg0h4fpavqe0owi1dm4yabio048.png)
The Outermost shell = 5s
so electron should be removed from 5s . there is only 1 electron in 5s -shell. Hence , Ag exist +1 oxidation state.
According to Aufbau's Rule : Electron will be filled first in lower energy orbital.
The electron should be removed first ,from the higher energy orbital.This is because high energy orbitals are away from the nucleus and can be easily removed. The outermost electron feel less attraction of the nucleus
Ionization energy : Energy required to remove the electron from the outermost shell of the element in the gaseous state.
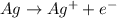
Hence it will form Ag+ cation
Ag+:
![[Kr]4d^(10)](https://img.qammunity.org/2021/formulas/chemistry/college/hnv1mdfycd9pdumtynzyhwq5182yfczci5.png)
Outer shell is removed after loss of electron.